Treatments for heart failure with reduced ejection fraction
Guideline-directed medical therapy (GDMT) treatment isn't always optimized
For patients with symptomatic heart failure, use of all 4 pillars of GDMT* was estimated to significantly reduce all-cause mortality. However, target doses may rarely be achieved in patients with heart failure with reduced ejection fraction (HFrEF) and may not be well tolerated when they are. This can limit the ability to slow disease progression, which may lead to worsening heart failure.1-3

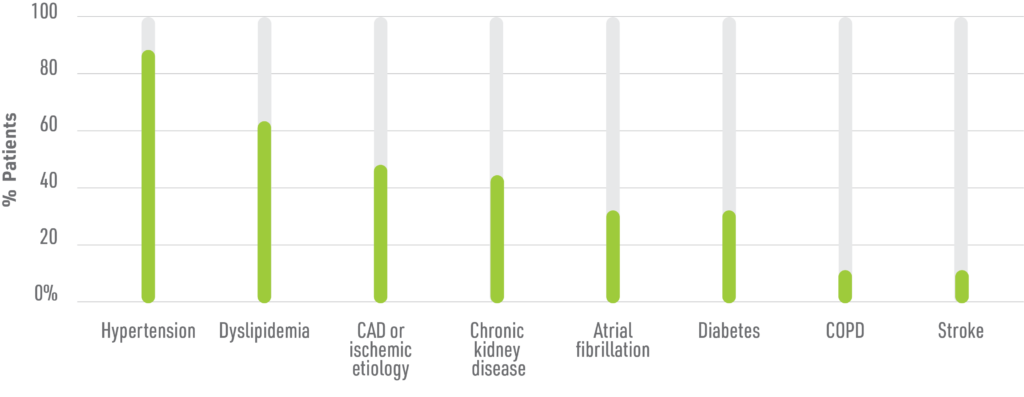
Patients with worsening heart failure often have multiple comorbidities, making GDMT optimization a challenge4
- Most patients with HFrEF typically have ≥3 comorbidities that require treatment4
- Often, patients with heart failure do not reach optimal GDMT doses due to tolerability issues2,3,5*
Adapted from Khan MS et al. Eur J Heart Fail. 2020.
Many GDMT options do not target cardiac contractility, which is a fundamental issue in HFrEF9,10
Current GDMT treatments that target neurohormonal pathways are rarely titrated to optimal doses in patients with HFrEF, due to side effects and tolerability issues
- These include declining renal function, higher risk of hyperkalemia, and hypotension3,11
However, there are treatment options that may directly impact cardiac contractility13
- Mitotropes focus on myocardial energetics
- Calcitropes are traditional inotropic agents that modulate calcium signaling
- Myotropes act upon the physical sarcomere
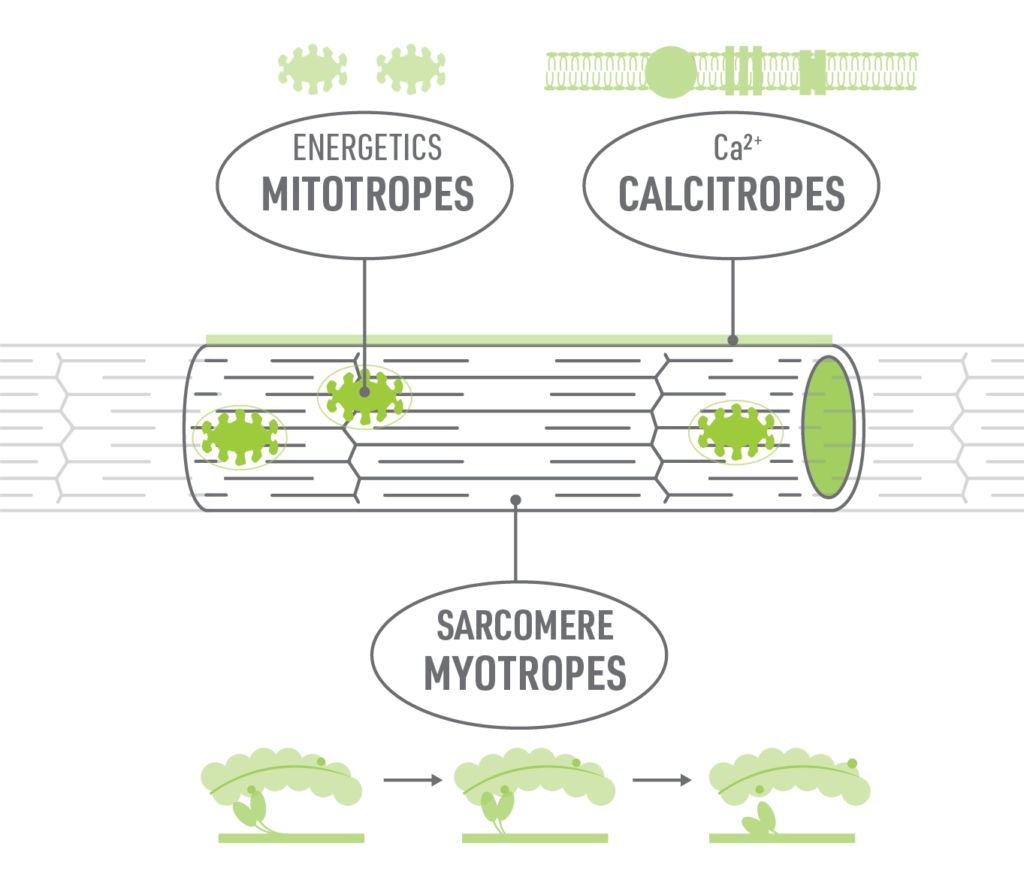
Since GDMTs for HFrEF act on neurohormonal pathways, another MOA that targets the sarcomere directly is being studied and may complement the treatment effects of current GDMT9,13
References: 1. Heidenreich PA et al. J Am Coll Cardiol. 2022;79(17):e263-e421. 2. Butler J et al. J Am Coll Cardiol. 2019;73(8):935-944. 3. Fiuzat M et al. JACC. 2022;79(5):504-510. 4. Saczynski JS et al. J Am Geriatr Soc. 2013;61(1):26-33. 5. Butzner M et al. Am J Manag Care. 2022;28(3):e113-e120. 6. Khan MS et al. Eur J Heart Fail. 2020;22(6):1032-1042. 7. Greene et al. Eur J Heart Fail. 2019;21(1):121-124. 8. Fry M et al. BMC Family Practice. 2016;17(139):1-8. 9. He H et al. Circ Heart Fail. 2022;15(3):e009195. 10. Mann DL et al. In: Longo DL et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. McGraw‑Hill; 2012:1901‑1915. 11. Verhestraeten C et al. Heart Fail Rev. 2021;26(6):1359-1370. 12. Unlu O et al. Circ Heart Fail. 2020;13(11):e006977. 13. Psotka MA et al. J Am Coll Cardiol. 2019;73:2345-2353.
